News
Search filters
DeepCLiDAR – Stage 2 Carbon Trust Certified Floating LiDAR
DeepCLiDAR, designed and built by CLS (a subsidiary of the French Space Agency), in partnership with the University of Maine, has obtained Stage 2 Carbon Trust Certification, making CLS a unique...
Woods Hole Group – Satellite Telemetry
At Woods Hole Group, Inc. we are the unique ARGOS satellite system operator in North America. ARGOS is a unique worldwide satellite location and data collection system dedicated to studying and...
Happy Holidays & Happy New Year!
We all know our planet is suffering, so let's get straight to the point. At Woods Hole Group, we have: data, continued services, expertise, value added proposals, people who care, to make our planet...
Debbie Stakem: A 32-Year Journey with Argos
An enhanced global system like Argos requires an enhanced global support team and, with its 30 different sites around the world, CLS is proud to have an excellent international userservices team....
Woods Hole Group Displays its Integrated Satellite Solutions for Sustainable Fisheries Management at Pacific Marine Expo 2023
The Pacific Marine Expo 2023, held in Seattle, Washington, is an exciting event for the commercial fishing industry, with numerous companies showcasing their innovations and solutions from across...
Can the Cape’s continually flooded streets be saved? A first look at possible solutions
On Cape Cod, low-lying roads are especially vulnerable to changes in sea level, whether churned up by storms or as a result of longer-term rise and erosion. With an eye toward making the region's...
NEMO VMS is Type-Approved for the American Lobster Fishery in the North Atlantic
NEMO VMS is type-approved by the Atlantic States Marine Fisheries Commission for the American Lobster Fishery in the North Atlantic.
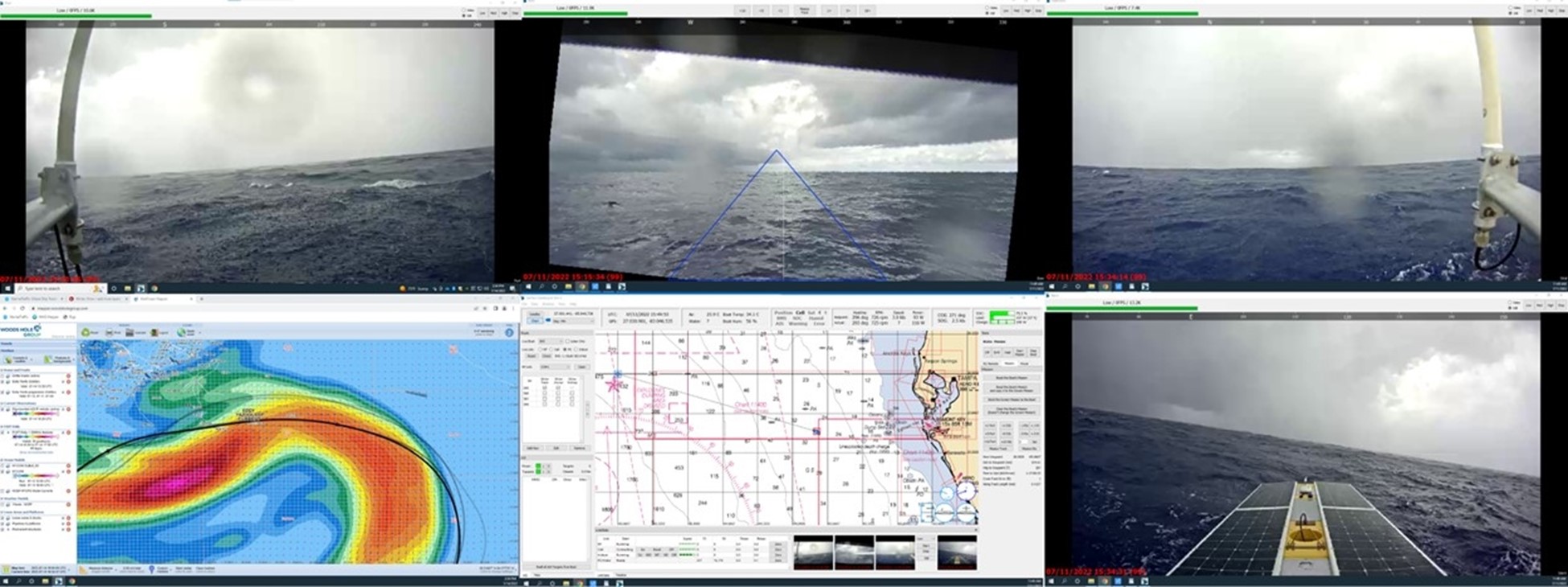
SeaTrac’s SP-48 completes 800 nautical mile offshore Loop Current monitoring mission in the Gulf of Mexico
SeaTrac Systems, Inc. (SeaTrac), in collaboration with the Woods Hole Group (WHG), successfully completed a 21 day over the horizon, deployment of SeaTrac’s SP-48 solar powered Uncrewed Surface...
Industry Focus: Woods Hole Group
Woods Hole Group solves environmental engineering problems worldwide with a focus on serving clients along the coast, in the ocean, and in wetland and terrestrial environments. Relying on service,...
Dune Restoration Study Funded For Stoney Beach In Woods Hole
Resilient Woods Hole, a group working to prepare Woods Hole for the impacts of climate change, has received state money to pay for a feasibility study for dune restoration at Stoney Beach. Although...
Community and Climate Risk in a New England Village
Woods Hole transformed from a whaling village to an ocean science research hub. Now, its next makeover involves a comprehensive climate change adaptation plan.Main Photo: Eel Pond (background left)...
Sea level rise threatens New Bedford’s fishing port and could put South Coast towns under water, new report finds
Rising seas along the South Coast are projected to have catastrophic effects, inundating towns around Buzzards Bay and Narraganset Bay, flooding out roads, and wiping away salt marshes that store...
New regulation in the Gulf Of Mexico
Based on the new NOAA/NMFS regulation, recreational charter-fishing vessels in the Gulf of Mexico have to be equipped with a Vessel Monitoring System in order to comply with local regulations. CLS...
Active Loop Current Drives Stellar 2021 FAST Eddy Season
In what has been a highly active year for operations in the Gulf of Mexico, a powerful Loop Current event threatened offshore assets with strong, dynamic ocean currents. In the face of unsafe...
Cape Cod Commission To Host Virtual Workshops On Low-Lying Roads Project
BARNSTABLE – The Cape Cod Commission recently announced that it is partnering with several Cape towns to educate the public in identifying at-risk roadways due to sea level rise in a series of...
Thomas Gray: Satellite Telemetry | Marine Animal Tracking
In this year’s final episode of SeaState, we are talking to Thomas Gray about Satellite Telemetry and Marine Animal Tracking. Thomas joined the Environmental Monitoring team at the Woods Hole Group...
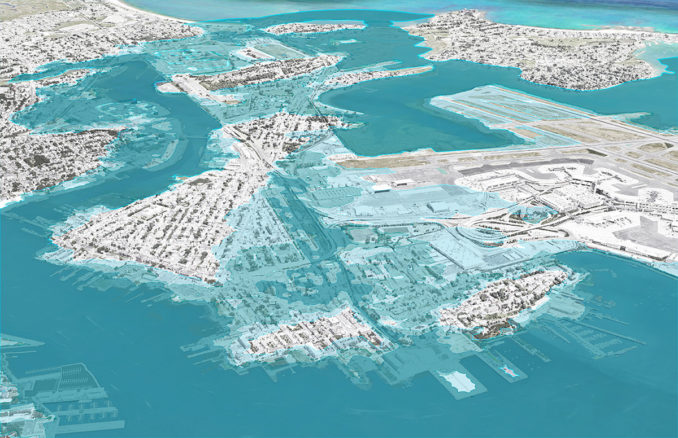
Palm Beach wants more than half a million dollars from state for climate change mitigation
Town officials are asking for more than half a million dollars from a newly created state coffer aimed at preparing Florida for the rising seas that come with climate change. The so-called Resilient...
Very Young Green Turtles Go Into the Sargasso Sea
The life of young (“lost years”) marine turtles had long been a mystery. Improvements in satellite telemetry now enable to unveil part of it. North Atlantic young green turtles, in particular, seem...
Three Top Tips for using your Goniometer with Thomas Gray
Thomas Gray joined the Argos team in 2016 and has quickly become our go-to person on all things Goniometer. He brings ten years of sales and marketing experience in the underwater tech realm...
Amid Climate Change Threats, Cape Planners Ask: Is It Time to Retreat from the Coast?
Catastrophic damage from climate change threatens coastal homes all over the Cape, and Islands, prompting regional planners to eye managed coastal retreat options. Whenever a beachfront home goes on...
David Walsh: Coastal Ocean Observing Systems
SeaTrac Systems, Inc. (SeaTrac), in collaboration with the Woods Hole Group (WHG), successfully completed a 21 day over the horizon, deployment of SeaTrac’s SP-48 solar powered Uncrewed Surface...
Wood Hole Group Announcement: Boston’s “Stone Living Lab” is Now Online!
Boston, MA – The Stone Living Lab is a new initiative which will engage researchers, students, and our communities in testing innovative, adaptive, and equitable nature-based approaches to climate...
The possibility of change in Marblehead Harbor
Woods Hole Group presented possible scenarios for climate change adaptation. When it comes to mitigating the impact of climate change on Marblehead Harbor, residents were hit with a lot of...
Woods Hole Group Offers Plans to Cope With Sea Level Rise In Falmouth
Building earthen berms in certain areas of Falmouth to protect vulnerable town infrastructure from flooding—and also creating a new harbor in Woods Hole—were among the ideas a Woods Hole Group...
With Nowhere to Hide from Rising Seas, Boston Prepares for a Wetter Future
Boston dodged a disaster in 2012. After Hurricane Sandy devastated parts of New Jersey and New York, the superstorm hit Boston near low tide, causing minimal damage. If Sandy had arrived four hours...
Coastal Resilience Solutions for East Boston and Charles-town | STOSS Landscape Urbanism
Coastal Resilience Solutions for East Boston and Charles-town outlines near- and long-term strategies to protect neighborhoods from flooding – in two of Boston’s most disadvantaged areas. It is the...
Introducing the new Woods Hole Group
FALMOUTH, MA – Representatives from CLS America, Inc., Horizon Marine, Inc. and The Woods Hole Group Inc. announced the merging of the three companies effective January 1, 2018 resulting in the new...
Massachusetts Hit Hard by Winter Storm
The winter storm that worked its way up the East Coast this week brought more than just snow to New England. Many communities along the coastline there are dealing with major flooding. From member...
USA: Woods Hole Group Wins Palm Beach Contract
Woods Hole Group, Inc., an international environmental, scientific, and engineering consulting organization based on Cape Cod, has been awarded a contract to review the Coastal Protection Plan for...